A groundbreaking gene therapy trial in France offers hope for treating a specific form of childhood deafness. The clinical trial, which just received approval in France, will assess the safety and efficacy of a new gene therapy drug in children aged between 6 and 31 months with profound hearing loss.
Audiogene, developed by the biotech company Sensorion, aims to treat children with DFNB9, a form of hereditary deafness caused by mutations in the OTOF gene.
- OTOF genes provide the instructions for synthesizing a protein called otoferlin.
- Otoferlin is essential for transmitting signals from the inner ear to the brain.
- The usual treatment for this form of hearing loss is a bilateral cochlear implant.
The success of this trial could pave the way for treating other forms of hearing loss and improve the quality of life for countless individuals struggling with this condition.
How it works:
- Audiogene (SENS-501) treats this childhood deafness by replacing the defective OTOF gene with a functional copy.
- The trial will test different doses and use advanced injection techniques to ensure accuracy and minimize risks.
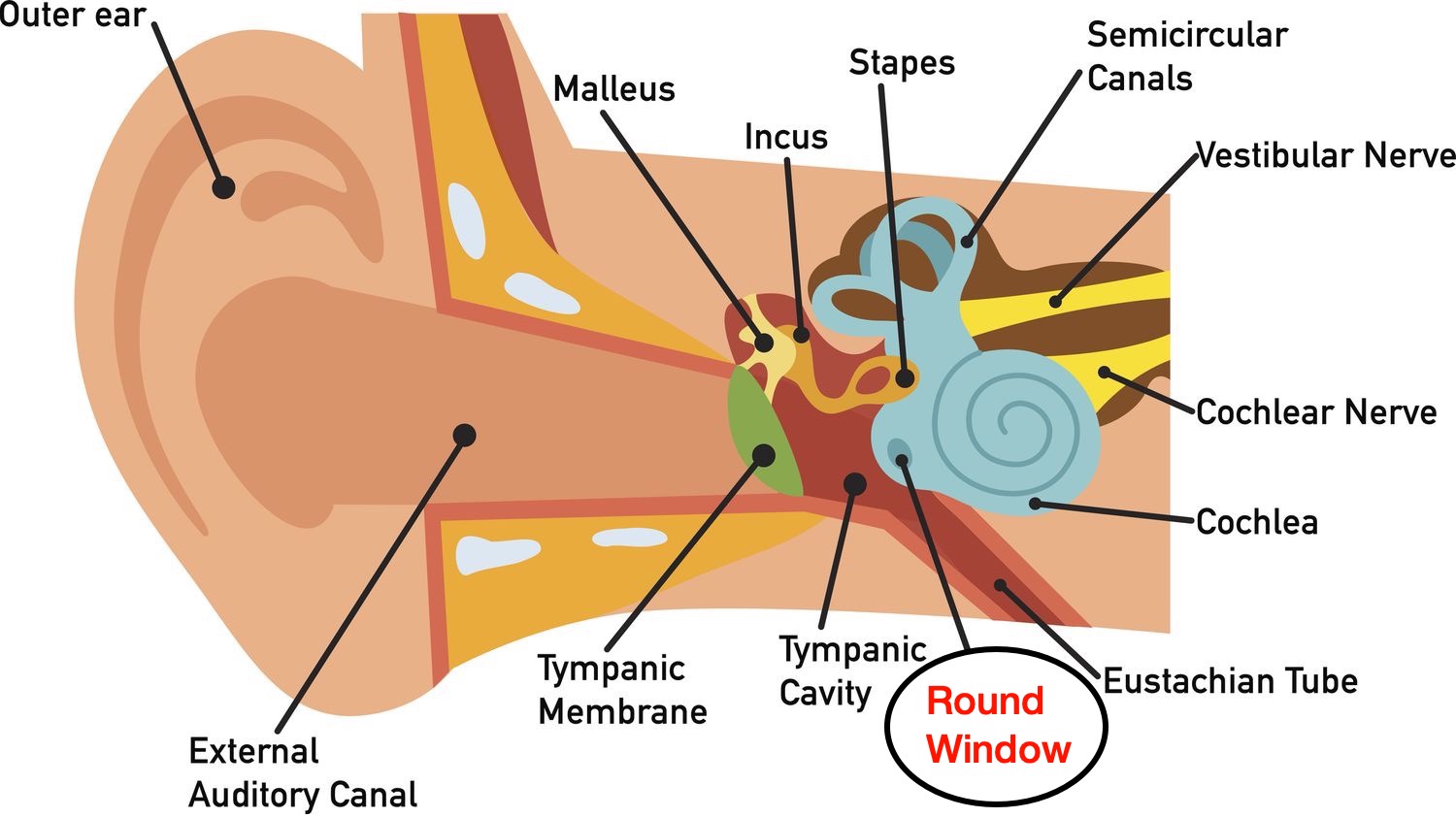 Anatomy of the inner ear. The Round Window is where the drug will be injected.
Anatomy of the inner ear. The Round Window is where the drug will be injected.
How it's done:
- The SENS-501 drug will be directly injected into the inner ear of the child with DFNB9 deafness using an injection system that accurately measures doses and preserves inner
- ear structures.
- The drug is injected into the round window in the inner ear like cochlear implantation surgery.
- The procedure will be performed under general anesthetic by an ENT surgeon.
Why it matters
This gene therapy has the potential to revolutionize hearing loss treatment.
- Millions of children suffer from hearing loss, often with limited treatment options.
- Restored hearing significantly improves learning, communication, social interaction, and overall quality of life for children with hearing loss.
What we're watching: We're staying tuned to this trial and will provide updates as soon as they're available.
1 big idea
This study represents a significant advancement towards a future where personalized gene therapy can restore hearing in children and adults.

