For some people with severe hearing loss, traditional cochlear implants don't work. Researchers in Switzerland have now developed a flexible brainstem implant that promises clearer sound with fewer side effects.
Why it matters
Superior design and clear sound. This soft, flexible implant offers hope for patients with damaged cochlear nerves who currently struggle with poor hearing quality from traditional brainstem devices.
“Designing a soft implant that truly conforms to the brainstem environment is a critical milestone in restoring hearing for patients who can’t use cochlear implants. Our success in macaques shows real promise for translating this technology to the clinic and delivering richer, more precise hearing." —Stéphanie P. Lacour, Head of the Laboratory for Soft Bioelectronic (LSBI) Interfaces at EPFL, Switzerland
The challenge
Current auditory brainstem implants (ABIs) have a critical flaw: their rigid design prevents them from fitting smoothly against the brainstem. As a result, doctors must disable most electrodes, preventing side effects like dizziness and uncontrolled facial movements. The consequence is devastating for patients—instead of hearing clear speech, they can only detect vague, indistinct sounds.
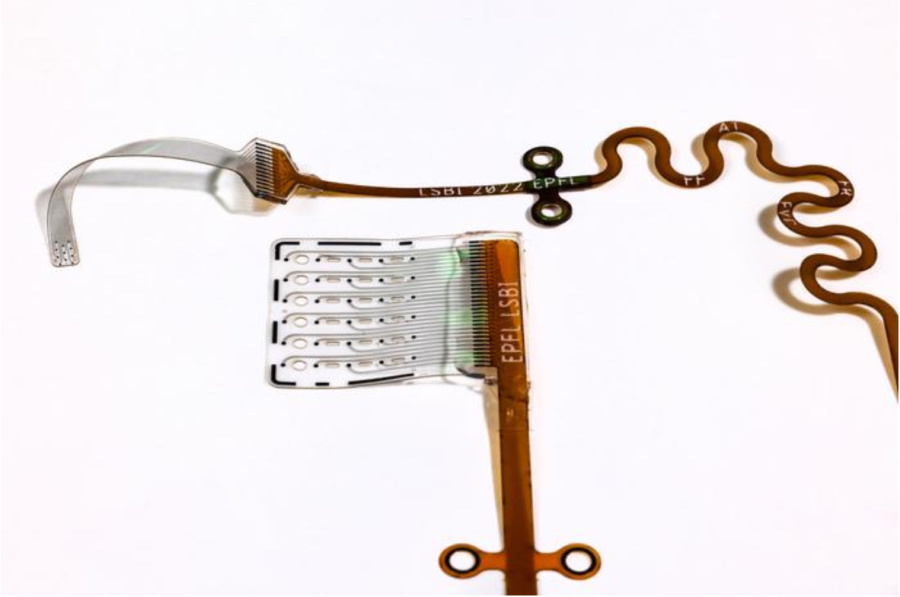 The soft auditory brainstem implant (ABI) developed at EPFL is designed to gently conform to brain tissue, enhancing signal precision and patient comfort.
The soft auditory brainstem implant (ABI) developed at EPFL is designed to gently conform to brain tissue, enhancing signal precision and patient comfort.
How it works
The new ABI bends like a sticker. The 11 platinum electrodes, embedded in ultra-thin silicone, mold to the brainstem’s curves. Better contact means fewer stray electrical currents—and fewer side effects.
“Our main idea was to leverage soft, bioelectronic interfaces to improve electrode-tissue match. If the array naturally follows the brainstem’s curved anatomy, we can lower stimulation thresholds and maintain more active electrodes for high-resolution hearing.” ” Alix Trouillet, PhD, co-first author of the study
By the numbers
- 11 electrodes in the current design, with potential for more.
- 1/3 millimeter thick—thinner than a credit card.
- 0 cases of facial twitching or discomfort in macaque trials.
Next steps
Human trials and ensuring long-term safety. If successful, this could redefine hearing restoration for thousands.
“One immediate possibility is to test the device intraoperatively in human ABI surgeries. They could briefly insert our soft array before the standard implant to measure if we truly reduce stray nerve activation.” —Dr. Lacour, referring to the team’s clinical partners in Boston, who regularly do ABI procedures for patients with severe cochlear nerve damage.


